Rametrix Technologies, Inc., announced today that they have developed a urine-based test for the presence of cancer in dogs. The announcement follows the publication of their study “Cancer detection in dogs using rapid Raman molecular urinalysis” in the prestigious, peer-reviewed journal Frontiers in Veterinary Science – Oncology. The study, co-authored...
News
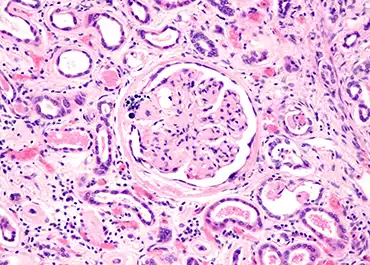
Diabetic Kidney Disease Detection
Analysis of urine Raman spectra differences from patients with diabetes mellitus and renal pathologies,” has been accepted for publication in the prestigious on-line journal, Peer J. This paper details the analysis of more than 250 urine specimens from Armed Services Veterans with our Rametrix® technology. An important finding from the...
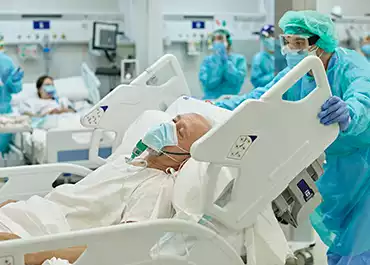
COVID-19 Hospitalized Patient Study
A study of 100 hospitalized COVID19 patient urine samples has shown that Rametrix® Molecular Urinalysis can be an effective tool in detecting kidney problems associated with infection. Early detection of COVID19 complications will allow healthcare providers to more effectively intervene with enhanced patient care.
Development of Controlled Substance Scanner
The ProofMed-CS V1.0 Scanner is in the final phase of engineering laboratory validation and certification. Once this is completed (February, 2023) user testing with key hospital pharmacy partners will begin. This use of this analytical device will enable real-time and point-of-care detection of unauthorized diversion of controlled substances in busy...
Advisory Board Testimonials
Rametrix® Technologies is pleased to announce the formation of their Business and Medical Advisory Board. See what some of our members are saying: Mike Abbott (January, 2023): “The application of technology to the improvement of the human condition is a remarkable and worthwhile endeavor and Rametrix® is poised to leave its...