Drug Checking
Analyze liquids & medications for purity and formulation accuracy in patient medication management and pharmaceutical & chemical industries.
Analyzing Medications for Patient Safety
Controlled Substance Diversion/ProofMed-CS™ Scanner V1.0
Criminal diversion of controlled substances (CS) in healthcare environments deprives patients of much-needed medications and is a serious and costly problem to manage. There are no current analytical methods to detect adulterated (diluted) in hospitals and clinics in real time and at the points-of-care. Our novel approach, the ProofMed™-CS Scanner V1.0, will facilitate rapid detection of adulterated CS injectables.

Substance Use Disorder
There is an epidemic of opioid misuse in the US and management of patients with SUD is difficult. Adherence to treatment programs and continued use of illicit (non-prescribed) drugs is problematic. Drug screens in current use simply are ineffective in monitoring patients and determining compliance with treatment. As well, these screens are not “real-time” (at the point of care and consultation), are expensive (hundreds of dollars) and do not detect wide potential variations in drug metabolism for individual patients.
Over an 18-month period, we will develop and validate a simple, non-invasive analytical technology, using serial urine and saliva samples from 100 consented patients (IRB Approval 22-1561, 10/19/22). These readily-available tests will be an aide to improving compliance with SUD management programs and for designing personalized, precision opioid treatment, based on individual patient pharmacokinetics. The use of this Raman spectroscopy-based, point-of-care technology during regularly-scheduled clinic visits, will facilitate “on the spot” decision making and treatment planning, as well as providing meaningful opportunities for counseling, and adjusting/reinforcing positive patient behaviors and lifestyle choices.
Chemotherapy Formulation/ProofMed™-CH Scanner V1.0
During treatment for cancer, patients receive multiple doses of highly toxic drugs. These drugs/doses may be ineffective or even more toxic if mixed or stored improperly, or compounded with substandard ingredients. Contamination of chemotherapy formulations with bacteria/fungi or endotoxins during mixing, while uncommon has sickened/killed patients. Because there is no on-site (Point-Of-Care) technology to assess dose composition, quality, formulation, and/or contamination, doses are very rarely checked – it is assumed doses are formulated correctly. If checked after formulation and immediately prior to administration, adverse patient reactions and deaths can be avoided. There is no competitor presently that can do it as efficiently as our proposed approach. There is clearly a need.
Using our proprietary Raman spectroscopy-based technology, we are creating an analytical device for real-time validation of proper formulation of parenteral cancer chemotherapy drugs by comparing formulated doses with reference chemotherapy standards and databases. The proposed device – ProofMed-CH™ Scanner V1.0 – will analyze 10 samples in a single 20-minute cycle.
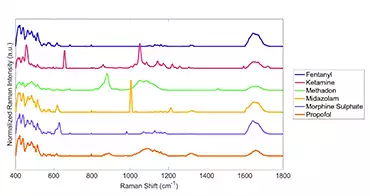
List of Substances Detected
- Morphine
- Fentanyl
- Methadone
- Suboxone
- Ketamine
- Propofol
- Xylazine
- Pentobarbital
Case Studies
Detection of diluted controlled substances using a computational Raman spectroscopy technology (Rametrix® Technologies, Inc.): Potential use in Point-Of-Care management of diversion…
WE ARE Rametrix® Technologies, Inc.
Every molecular fingerprint matters!
