Controlled Substance Diversion in Healthcare Facilities
Controlled Substances (CS) are essential for anesthesia and pain management in every hospital (target market – 6093 hospitals, six POCs/hospital). CS use is highly regulated (DEA, state agencies) to prevent misuse or criminal ‘diversion’ (theft). CS diversion is widespread; in 2019, >148M doses were diverted. According to the Joint Commission on Resources, many hospitals do not meet or have lax, inefficient enforcement of medication security standards. Injectable CS (e.g., morphine, fentanyl) were diluted/replaced/wasted/’lost’ at many points of transit from pharmacies to, or at, high-risk POCs (emergency depts, endoscopy, oncology, ob-gyn, post-surgical ICU). Diversion by staff (including doctors and nurses with addictions) has been documented. Inadvertent administration of adulterated, contaminated CS to hospitalized patients has resulted in deaths.
DEA penalties for CS misuse/diversion are severe: up to $68,000 per medication violation. According to pharmacy managers, millions of dollars are invested annually in CS inventory programs, dispensing and unit dosing systems, ‘smart technology’ (HD cameras/facial recognition) in pharmacies/storage sites, and oversight of staff administering CS.
We, at Rametrix® Technologies, Inc. analyzed current hospital procedures for preventing CS diversion. During customer discovery, we identified a critical point of diversion (POC disposal) not addressed by current CS practices or technology. CS not used by healthcare personnel for patients at the POC are required, by law, to be inventoried (measurement of unused volume of drug) and ‘wasted’ (flushed in a toilet, for example) or destroyed by other secure means. Destruction of unused or expired CS is required to be impartially witnessed. Unobserved ‘skillful’ adulteration (removal of CS, replacement with other fluids) can be easily accomplished before or at POC disposal. Chemical analysis of CS at the POC is rarely done voluntarily or is operationally impractical; current methods (measuring specific gravity) doesn’t detect ‘skillful’ (criminal) adulteration.
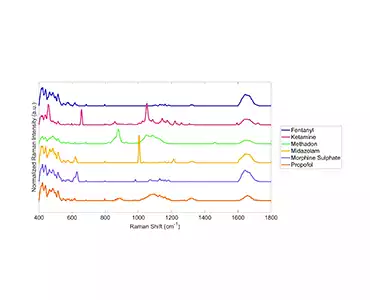
As a proof-of-concept, we (under supervision, of course!) obtained samples of commonly diverted CS, intentionally diluted them from 100%-1% and then analyzed the samples using our patented Rametrix® Technologies, Inc. technology. The results of this work are available as a Resource and are currently being prepared for publication.
Based on the success of our proof-of-concept testing, we designed and built a semi-automated system (ProofMed-CS™ Scanner V1.0) for CS measurement/analysis/destruction at the POC. We have tested and patented the Raman analysis (Rametrix® Technologies, Inc.) and measurement technologies (AutoScanner V2.0™) for molecular analysis that we applied to the problem of CS POC disposal management. Our system will use disposable cassettes for ‘one stop measurement/analysis/destruction’ of CS.
There are no competitors in CS POC disposal management.